
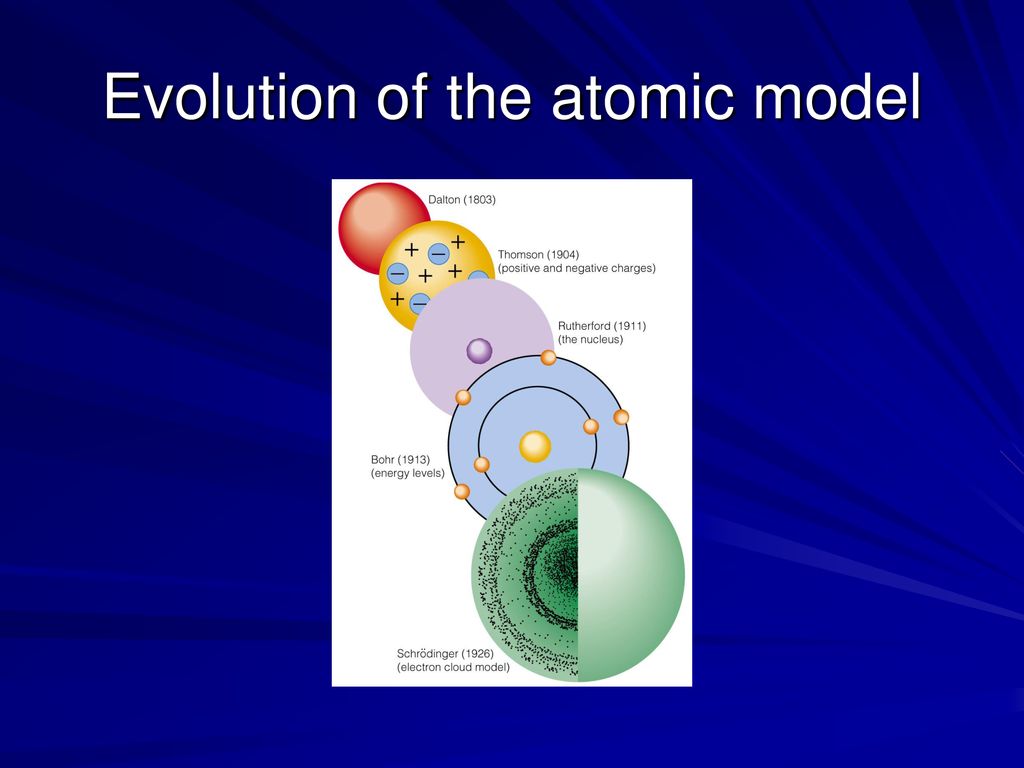
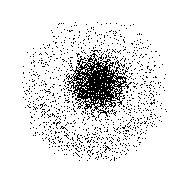
Various atoms and molecules as depicted in John Dalton’s A New System of Chemical Philosophy (1808). However, this theory was more of a philosophical concept than a scientific one. The term “atom” was coined in ancient Greece and gave rise to the school of thought known as “atomism”. The earliest known examples of atomic theory come from ancient Greece and India, where philosophers such as Democritus postulated that all matter was composed of tiny, indivisible and indestructible units. Hence, their locations could only be described as being part of a ‘cloud’ around the nucleus where the electrons are likely to be found. Instead, Schrodinger proposed a model whereby scientists could only make educated guesses as to the positions of electrons. Thanks to this model, electrons were no longer depicted as particles moving around a central nucleus in a fixed orbit. One such example is the Electron Cloud Model proposed by Erwin Schrodinger.

Thanks to ongoing studies on the behavior of electrons, scientists began to propose theories whereby these elementary particles behaved in ways that defied classical, Newtonian physics. In addition to Ernest Rutherford and Niels Bohr giving birth to the Standard Model of particle physics, it was also a period of breakthroughs in the field of quantum mechanics. The early 20th century was a very auspicious time for the sciences.


 0 kommentar(er)
0 kommentar(er)
